The Intricate Palette of Gemstone Colors: A Deep Dive
The realm of gemstone colors is a kaleidoscope, far beyond the simplicity of naming shades. Terms like “pigeon’s blood”, “Paraíba”, and “cobalt blue” have woven themselves into the fabric of the gem world’s lexicon, attesting to the richness and diversity of hues that nature bestows upon these earthly treasures. Yet, the bridge between coining such evocative terms and their consistent, rational application across the gemstone industry spans a vast and complex chasm.
Beauty in the Eye of the Beholder: The Subjectivity of Color
The quest for the most beautiful color—a blue more splendid than the sky on a clear day, or a red that burns brighter than the sunset—is a pursuit mired in personal preference. Social media, with its myriad of self-proclaimed color experts, often sees assertive declarations about the “right” hue of a gemstone, based purely on a glance at a digital image. This digital discourse, however, merely skims the surface of the profound complexity inherent in describing gemstone colors.
Paraíba: The Luminous Wonder

In 1982, the discovery of vibrant blue gemstones in the pegmatite deposits of Paraíba, Brazil, by Heitor Dimas Barbosa, marked the beginning of a new chapter in the story of tourmalines. It wasn’t until 1983, however, that the source of these mesmerizing stones was pinpointed, fundamentally transforming the global tourmaline market. The unique blue-green hue of Paraíba tourmalines, enriched by copper (lending a blue tone) and manganese (adding a touch of green), spans a spectrum from emerald green to sapphire blue, with dazzling shades in between. Terms like “electric” and “neon” have been coined to capture the essence of Paraíba tourmalines’ unparalleled vibrancy.
By 1999, a similar type of tourmaline was discovered in Nigeria, exhibiting comparable colors but lacking the intense neon glow of its Brazilian counterparts. Differences in chemical composition, notably higher lead content in the Nigerian Paraíba, serve as a distinguishing factor between the two. The subsequent discovery of Paraíba-like tourmalines in Mozambique in 2001 added another layer of intrigue. These Mozambican gems closely mirror the Brazilian Paraíba in color and composition, yet they stand out for their clarity and size, attributes that the Brazilian stones seldom match.
According to the LMHC (Laboratory Manual Harmonisation Committee), Paraíba tourmalines are defined by their electric blue, neon blue, violet blue, bluish-green, greenish-blue, green, and yellowish-green hues. The saturation and tone, ranging from light to medium, are primarily due to the presence of copper and manganese, irrespective of geographical origin. This classification underscores the unique coloration that defines Paraíba tourmalines, distinguishing them from other varieties lacking in copper content or outside the specified color range.
Cobalt Blue: The Quest for the Ultimate Blue
Cobalt has long been a staple in coloring synthetic spinel, creating shades of blue that captivate the eye. However, it was not until the early 1980s that gemologists identified cobalt as a natural colorant in blue gemstones from Sri Lanka. This discovery was followed by the appearance of cobalt blue spinel in Vietnam and Tanzania, expanding the palette of natural blue gemstones. Yet, not all natural blue spinels contain cobalt, prompting a debate over what truly deserves the “cobalt blue” label. Recent research suggests that spinels ranging from blue to violet may owe their hues to a variety of color-causing elements, including Co2+ for blue, Fe2+ and Fe3+ for greenish to blue tones, Cr3+ for red, and V3+ for orange, with Mn2+ and/or Mn3+ possibly playing a role as well.

Chromophores: The Elemental Palette
Using elemental colorants to separate gemstone types may not necessarily solve the problem. Take emeralds, for example. For over a century, emeralds were “defined” by gemologists as green beryls colored by chromium, in contrast to “green beryls.” Later, it was discovered that many “emeralds” are actually colored by vanadium. And let’s not forget “chrome tourmaline,” which is also partially colored by vanadium.
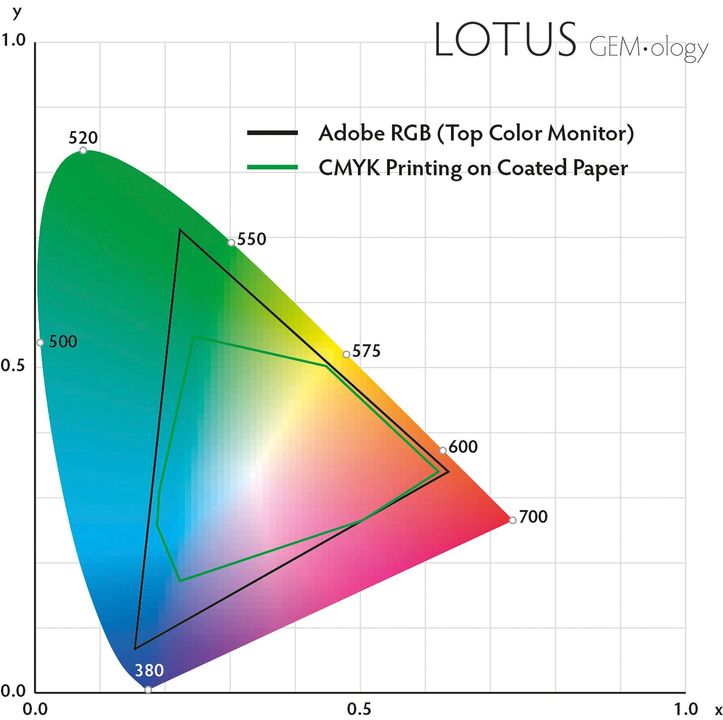
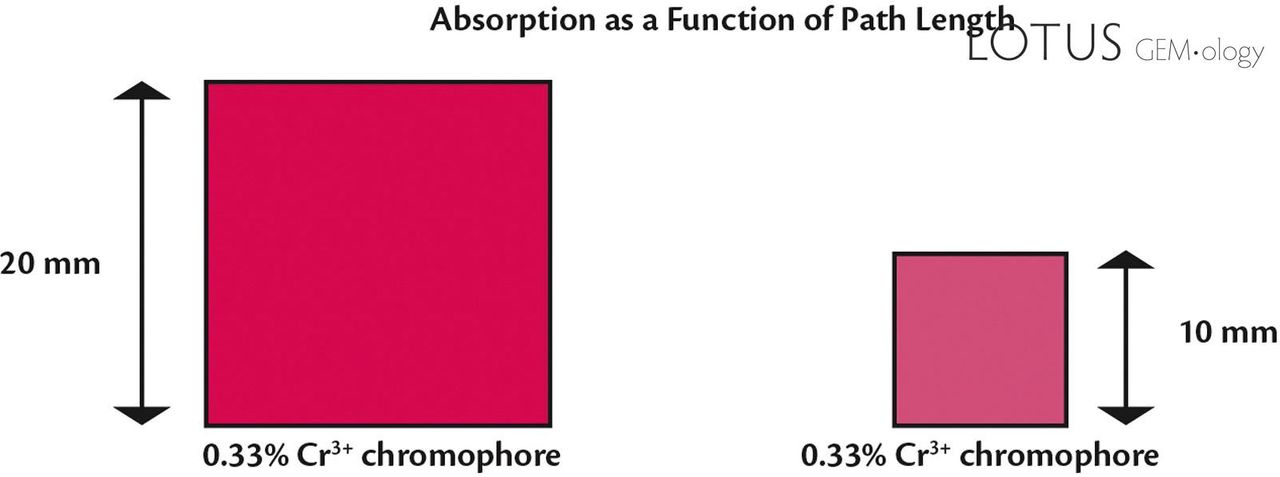
The reality is that our current understanding of chromophores is imperfect. Therefore, defining a type of stone based on the colorant element may not be accurate. The stone itself does not change; only the definition changes. The color of colored gemstones is often the result of mixtures of different colorant elements. Even with a single colorant element, the color will vary because it is produced by the partial absorption of the spectrum of white light, and the remaining light reflected to our eyes is a selective color. This absorption also depends on the path length of white light passing through the material. This is why defining a type of gemstone based on the percentage ratio of any colorant element is very difficult.
For example, if you cut a 10 mm synthetic ruby square slice and a 20 mm synthetic ruby square slice from the same raw stone block, the 20 mm square slice will have a darker color than the 10 mm slice, even though they have the same percentage ratio of chromium. This is because the path length of white light in the two different slices is different.
In 2015, Stuart Robertson of Gemworld surveyed over 500 rubies listed on the Gemstone Trading Network Polygon (Robertson, 2023, pers. comm.). More than 90% of the certified rubies were of a “pigeon’s blood” color. A similar situation occurred in the early 20th century when almost all diamonds sold on the market were labeled “blue-white.” This prompted the United States Federal Trade Commission in 1938 to require the prohibition of using this term because it was deemed “unfair trade practice.”
A Practical Illustration: The Ruby Example
Consider two synthetic ruby slices cut from the same rough stone: one 10 mm thick and the other 20 mm. Despite having the same percentage of chromium, the 20 mm slice appears darker due to the longer path light travels through it. This phenomenon underscores the difficulty of defining a gemstone’s color by its chromophore content alone.
In 2015, Stuart Robertson of Gemworld surveyed over 500 rubies listed on the gemstone trading network Polygon. He found that more than 90% of the rubies accompanied by certification were described as “pigeon’s blood” red. This situation mirrors the early 20th century when almost all diamonds on the market were labeled “blue-white,” leading the Federal Trade Commission in 1938 to ban the term as “unfair trade practice” for its misleading nature.
The Color Complexity in Gemstones
The popularity of color terms like padparadscha, pigeon’s blood, royal blue, and cobalt blue, while widespread, often leads to debates among gemologists, traders, and jewelers due to a lack of consistency. This inconsistency is not surprising given the following characteristics of colored gemstones:
- They can exhibit pleochroism, displaying multiple colors.
- Color zoning significantly affects their overall hue.
- Visible impurities and differences in texture impact their appearance.
- Their color can change under different lighting conditions.
- They are often cut asymmetrically.
- Even symmetrically cut colored gemstones do not display a uniform color throughout.
The Quest for a Standard Color Chart
Gemologists turn to various color charts as guides for describing gemstone colors, including GemGuide’s World of Color, AGL’s ColorCodex, and the Munsell Color System, among others. However, these systems offer at best a crude reference, primarily because they deal with color as a monochromatic image. None can fully replicate the vast universe of colors, intensities, hues, and tones found in natural colored gemstones.
Conclusion
In the vast and intricate realm of gemstone colors, where terms like “pigeon’s blood” and “cobalt blue” evoke vivid imagery, lies a complex landscape of subjectivity and variability. The pursuit of defining and categorizing these hues spans from the mesmerizing Paraíba tourmalines to the elusive cobalt blue spinels. Yet, amidst the quest for standardization, the subjective nature of color perception, influenced by factors like lighting and impurities, persists. From the enigmatic chromophores to the challenges of creating a standard color chart, the journey to codify gemstone colors is fraught with nuances. In this kaleidoscope of color, consistency remains an elusive yet worthy endeavor for gemologists, traders, and enthusiasts alike.


